Past Issues
Effectiveness of Therapeutic Plasma Exchange in a Patient of Myasthenia Gravis with an Atypical Presentation: A Case Report
Satya Prakash1*, Gunjan Kumar2
1Department of Transfusion Medicine, All India Institute of Medical Sciences, Bhubaneswar, Odisha, India
2Assistant Professor, Department of Neurology, All India Institute of Medical Sciences, Patna, India
*Corresponding author: Dr. Satya Prakash Department of Transfusion Medicine, All India Institute of Medical Sciences, Bhubaneswar, Odisha, India. Tel: +918872533400. E-mail: [email protected]
Citation: Prakash S, Kumar G. (2022). Effectiveness of therapeutic plasma exchange in a patient of myasthenia gravis with an atypical presentation: a case report. Cases. 1(1):1.
Received: April 23, 2022
Published: November 04, 2022
ABSTRACT
The grave muscle weakness in Myasthenia is attributed to circulating auto-antibodies against the nicotinic acetylcholine receptor, muscle-specific kinase, agrin, and others. Patients with myasthenia generally present with fatigue and drooping of eyelids, diplopia, and slurred speech. Sensory involvement is rare in Myasthenia Gravis. Most of these patients respond very well to steroids and other immunomodulatory drugs. Therapeutic plasma exchange (TPE) is used as first-line therapy to treat these patients presenting with the crisis. Here we report TPE in a case of myasthenia patients in crisis presenting with no eyeball movement in any gaze, mild dysphagia, and slurred speech. The patient underwent a total of 4 TPE procedures and in the end, she had improved diplopia with no ptosis and only a slightly nasal twang. This report emphasizes the role of TPE in atypical myasthenia with sensory involvement where the diagnostic dilemma exists.
Keywords: Plasma Exchange, Auto-antibody, Muscle weakness, Nicotinic acetylcholine receptor
INTRODUCTION Myasthenia gravis is an autoimmune disease characterized defect in neuromuscular transmission resulting in progressive neuromuscular weakness of bilateral upper and lower limb. Consequently, it involves extraocular muscles of the eye resulting in ptosis, diplopia, restricted lateral gaze and finally, involves bulbar part resulting in dysphagia, slurred speech and then respiratory failure [1]. Disease signs and symptoms were explained by the presence of autoantibodies against acetylcholine receptor, muscle-specific tyrosine kinase receptor, and many others like low-density lipoprotein receptor-related protein 4 [2,3]. Although serology had an important role in diagnosis still even seronegative patients got benefitted from therapeutic plasma exchange, which prompts the researcher to search for a more sensitive test or for test of another antibody against agrin, collagen q and cortactin [4,5].
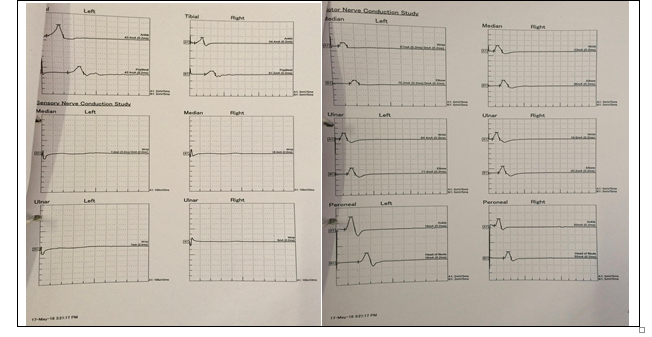
CASE REPORT Here, we present a case of myasthenia gravis with an atypical presentation in the form of sensory involvement along with the typical presentation of bilateral both upper and lower limb weakness (movement only with support), ptosis, restricted eye movement in all gaze, mild dysphagia with solid food and slurred speech with a nasal twang. The patient had all these signs and symptoms for the last seven years and on various immunosuppressants, but symptoms only worsen slowly with no improvement in muscle power in both the upper and lower limb. The patient was referred to our Centre from a tertiary care Centre for further evaluation and appropriate management. On the basis of previous history and investigation, the nerve conduction study was performed, which shows sensorimotor involvement (Figure 1). After NCS, the diagnosis was not clear and mentioned as a suspicious case of a myasthenic crisis/mitochondrial myopathy. The Chest Computerized tomography scan also excludes any possibility of thymoma. All other hematological and biochemical parameters were found to be normal, as mentioned in table 1. However, in view of symptoms and signs in favor of myasthenic crisis and borderline anti-AchR Antibody, therapeutic plasma exchange was planned.
TPE was done with Cobe spectra version 7.0 (Terumo BCT, USA) on an alternate day basis with normal saline and 5% human serum albumin as replacement fluid. After the first TPE, the patient improved in the form of mild eye-opening (Ptosis improved), speech and eye movement also improved in all gaze. A total of four TPE procedures were performed with an average volume of plasma exchange per procedure was 1500 ml (equivalent to one plasma volume of the patient). The patient had an allergic reaction to human serum albumin during the first TPE procedure in the form of rashes and urticaria on face, neck and abdomen, which subside spontaneously in 20 minutes with injectable intravenous chlorpheniramine 25mg and hydrocortisone 100mg. The next three TPE procedures were done with premedication with injectable intravenous chlorpheniramine 25mg and hydrocortisone 100mg in view of an allergic reaction during the first TPE and all four procedures were completed successfully without any other adverse event. Continuous calcium gluconate intravenous infusion (10ml per 1000ml of plasma exchanged) was given during the procedure prophylactically to counteract the citrate effect due to the use of acid citrate dextrose-A as an anticoagulant.
Figure 1: Showing mainly sensory involvement (Median and ulnar nerve) in nerve conduction study
Table 1: Showing Hematological and Biochemical parameter of the patient during the course of TPE
|
Investigations |
At Admission before TPE |
After 4th TPE |
At discharge |
|
Hb |
130 gm/L |
121gm/L |
120 gm/L |
|
TLC |
9.8x109/L |
10.1x109/L |
11.3x109/L |
|
PLT |
150x109/L |
203x109/L |
279x109/L |
|
Na |
144.0 meq/L |
137.9 meq/L |
132.7 meq/L |
|
K |
4.0 meq/L |
4.3 meq/L |
4.5 meq/L |
|
Cal |
9.83 mg/dl |
8.58 mg/dl |
8.7mg/dl |
|
Ion Cal |
0.84mmol/L |
1.17mmol/L |
1.04mmol/L |
|
Alkaline Phosphatase |
52 U/L (normal 46-116 U/L) |
|
|
|
CPK |
328 U/L (normal <140 U/L) |
|
|
|
Anti-achR antibody |
0.29ng/ml (0.15-0.31ng/ml) |
|
|
Table 2: Showing signs and symptoms of patient during course of TPE
|
Sign and symptoms |
Muscle power |
Ptosis |
Slurred speech With Nasal twang |
Eye movement |
|
At admission |
3/5 |
Severe |
Present |
Restricted in all gaze |
|
After 4th TPE |
5/5 |
Mild |
Improved but slightly present |
Present in all gaze |
|
At discharge |
5/5 |
No Ptosis |
Improved but slightly present |
Improved and present in all gaze |
Thus, there was a dilemma in the diagnosis of myasthenia gravis due to sensory involvement with fixed eyeball (movement restricted in all gaze) along with similar signs and symptoms of myasthenia gravis. Still, TPE was done as a last resort and the patient had significant improvement in muscular weakness, eye movement in all gaze and improved speech along with no ptosis.
DISCUSSION Therapeutic plasma exchange is indicated as first-line treatment for the patient with myasthenic crisis [6]. The overall response rate after TPE was satisfactory in a maximum number of myasthenia patients (81.8%) as quoted by study of Sharma et al [7]. However, only few studies quote for the presence of sensory deficit along with motor involvement in myasthenic patients but still, dilemma persists [8]. In our patient, the sensory nerve conduction study clearly showed a very low amplitude, as shown in figure 1 and is not typical of myasthenia. This patient also had eye movement nearly completely restricted in all gaze, which is also a rare finding as partial ophthalmoplegia commonly occurs due to extraocular muscle weakness involving medial rectus commonly followed by superior rectus [9]. Restricted eye movement gets resolved partially after the first procedure and completely after 4th TPE. The rest of the feature is similar to other patients of myasthenia like ptosis, dyspnea and bilateral limb weakness. A similar spectrum of signs and symptoms was also associated with progressive external ophthalmoplegia due to mitochondrial myopathy but TPE had no role in the management thus, again, diagnosis is in favor of myasthenia gravis [10]. Miller fisher syndrome presents with triad of acute ophthalmoplegia, ataxia and areflexia along with ptosis and diplopia [11,12]. Our patient also had ptosis, diplopia and ophthalmoplegia but areflexia and ataxia was not present and the onset of symptom was also subacute and slowly progressive, not responding to immunosuppressive therapy. Progressive muscle weakness in Polymyositis primarily involves proximal muscles but facial and ocular muscles were spared [13]. Inspite of various atypical presentations, TPE was performed with a provisional diagnosis of myasthenia crisis and all the signs and symptoms were resolved with TPE progressively and finally, the patient got discharged from the hospital after 4th procedure. The patient had an allergic reaction during first TPE evident as rashes, hives and urticaria over face, neck and abdomen.
Albumin may be the culprit here as no other intravenous fluid or medicine was given during the procedure. Many reports suggested albumin infusion as cause of allergy or anaphylaxis. However, albumin is favored for TPE as there is a lesser risk of transfusion-transmitted infections as well as very rarely it causes an allergic reaction in patients [14].
CONCLUSION Therapeutic plasma exchange can be used as a last resort treatment modality in drug-resistant suspected cases of myasthenia with atypical presentation and uncommon sensory deficit at presentation. RECOMMENDATION TPE can be used as a last resort modality in patients with atypical myasthenia where diagnostic dilemma exists due to overlapping clinical features with borderline serology test results.
FUNDING None
CONFLICT OF INTEREST None
REFERENCES
1. Gilhus NE, Verschuuren JJ. (2015). Myasthenia Gravis: Subgroup classification and therapeutic strategies.Lancet Neurol. 14(10):1023-1036.
2. Cetin H, Vincent A. (2018). Pathogenic mechanisms and clinical correlations in autoimmune mysthenic syndromes. semin neurol. 38(3):344-354.
3. Sieb JP. (2014). Myasthenia gravis. An Update for Clinician. Clin exp Immunol. 175(3):408-418.
4. Gilhus NE, Skeie GO, Romi F, Lazaridis K, Zisimopoulou P, Tzartos S. (2016). Myasthenia gravis-autoantibody characteristics and their implications for therapy. Nat Rev Neurol. 12(5):259-268.
5. Prakash S, Hans R, Sharma RR, Lal V, Marwaha N. Therapeutic plasma exchange in a patient of myasthenic crisis, refractory to intravenous immunoglobulin and immunosppressive therapy. Neurol India. 65(6):1409- 1412.
6. Padmanabhan A, Connelly-Smith L, Aqui N, Balogun RA, Klingel R, Meyer E, et al. (2019). Guidelines on the Use of Therapeutic Apheresis in Clinical Practice - EvidenceBased Approach from the Writing Committee of the American Society for Apheresis: The Eighth Special Issue. J Clin Apher. 34(3): 171-354.
7. Sharma RR, Saluja K, Jain A, Dhawan HK,Thakral B, Marwaha N. (2011). Scope and application of therapeutic apheresis: Experience from a tertiary care hospital in north in India. Transfus Apher Sci. 45(3):239-245.
8. Fidias E. Leon-Sarmiento, Juan S. Leon-Ariza, Prada D, Leon-Ariza DS, Rizzo-Sierra CV. (2016). Sensory aspects in myasthenia gravis: A translational approach, J Neurol Sci. 368:379-388.
9. Nair AG, Patil-Chabblani P, Venkatramani DV, Gandhi RA. (2014). Ocular myasthenia gravis: A review. Indian J Ophthalmol. 62(10): 985-991.
10. Pfeffer G, Chinnery PF. (2013). Diagnosis and treatment of mitochondrial myopathies. Ann Med. 45(1): 4-16.
11. Anthony SA, Thurtell MJ, Leigh RJ. (2012). Miller fisher syndrome mimicking ocular myasthenia gravis. Optom Vis Sci. 89(12): e118–e123.
12. Borges LS, Richman DP. (2020). Muscle-Specific Kinase Myasthenia Gravis. Front Immunol.11:707.
13. Gazeley DJ, Cronin ME. (2011). Diagnosis and treatment of idiopathic inflammatory myopathies. Ther Adv Musculoskelet Dis. 3(6): 315-324
14. Gales BJ, Erstad BL. (1993). Adverse reactions to human serum albumin. Ann Pharmacother. 27(1):87-94.
Copyright: Prakash S. © (2022). This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
 Abstract
Abstract  PDF
PDF