Past Issues
The Impregnated Integument- Cutaneous Marginal Zone Lymphoma
Bajaj A*
Consultant Histopathologist, AB Diagnostics, New Delhi, India
*Corresponding author: Dr. Bajaj Anubha Consultant Histopathologist, AB Diagnostics, New Delhi, India. Tel: 91-98116-93956. E-mail: [email protected]
Citation: Bajaj A. (2022). The Impregnated Integument- Cutaneous Marginal Zone Lymphoma. Cases. 1(1):2.
Received: November 02, 2022 Published: November 12, 2022
Copyright: Bajaj A © (2022).
Primary cutaneous marginal zone B cell lymphoma (CMZL) is an indolent lymphoma demonstrating nonspecific post-germinal centre B cell phenotype. The B cell lymphoma is derived from mature, predominantly post-germinal centre B lymphocytes. World Health Organization (WHO) classifies the cutaneous B cell lymphoma as an extra-nodal marginal zone B cell lymphoma of mucosa-associated lymphoid tissue (MALT). Thus, primary cutaneous marginal zone lymphoma is additionally designated as extra-nodal marginal zone B cell lymphoma of mucosa-associated lymphoid tissue (MALT) and configures approximately 30% of cutaneous B cell lymphomas. Primary cutaneous marginal zone lymphoma frequently incriminates young to middle-aged subjects whereas the lymphoma is exceptionally discerned within paediatric population [1,2]. Primary cutaneous marginal zone lymphoma commonly emerges within trunk or upper extremities although sites of disease occurrence are variable. Lesions are predominantly solitary, localized or regional. Few lesions may be generalized or disseminated. The infrequent extra-cutaneous dissemination may be indicative of cutaneous dissemination of an extra-cutaneous lymphoma, possibly a splenic or nodal lymphoma [1,2]. Of obscure aetiology, primary cutaneous marginal zone lymphoma is posited to arise consequent to cutaneous lymphoid hyperplasia engendered due to diverse antigenic triggers [1,2]. Chronic antigenic stimulation may induce immune dysregulation with subsequent occurrence of cutaneous marginal zone lymphoma. Clonal rearrangement of immunoglobulin heavy chain (IGH) concurs with primary cutaneous marginal zone lymphoma. Nevertheless, infiltrating inflammatory cells may predominantly be comprised of reactive T lymphocytes and B lymphocytes [1,2]. Trisomy 3 (60%) is accompanied by a subset of neoplasms demonstrating trisomy 7, trisomy 12 or trisomy 18. Chromosomal translocation t(11;18)(q21;q21) with consequent API2-MALT1 genetic fusion may exceptionally ensue [1,2]. Chromosomal translocation t(14;18) with genetic metamorphosis of IGH / BCL2 or IGH / MLT1 may be discerned infrequently. Besides, chromosomal translocation t(3;14) with IGH - FOXP1 genomic fusion is documented. A subset of primary cutaneous marginal zone lymphomas exhibits chromosomal 7q deletion associated with metamorphosis into an aggressive blastic phenotype [1,2].
Primary cutaneous marginal zone lymphoma manifests distinct subcategories as • non-class switched lymphoma which recapitulates extracutaneous marginal zone lymphoma as it demonstrates cellular inflammation with chronic Th1 lymphocytes along with enhanced production of Th1-associated cytokines including interferon-gamma (IFN?) and interleukin 2(IL2). Besides, Borrelia burgdorferi antigen may be discerned in a subset of aforesaid lymphoma occurring in Europe [1,2]. • heavy chain class switched lymphoma configures a majority of cutaneous marginal zone lymphomas. Upon histological assessment, the neoplasm is distinct from extra-cutaneous marginal zone lymphoma and arises in association with chronic inflammation constituted by Th2 lymphocytes or preponderant reactive T and B lymphocytes. Besides, the variant may arise within individuals delineating an allergic or atopic diathesis. Certain instances may occur due to medications such as methotrexate, cyclosporine, antidepressants or antihistamines. Cutaneous marginal zone lymphoma may concur with infectious diseases arising due to Helicobacter pylori, Hepatitis C, and Epstein Barr virus [1,2].
Autoimmune diseases such as polyarteritis nodosa, Sjögren’s syndrome, rheumatoid arthritis, Hashimoto’s thyroiditis, or ulcerative colitis may appear concordant with cutaneous marginal zone lymphoma. Repetitive mutations within FAS gene which incriminate death domain of apoptosis regulating FAS / CD95 protein may be associated with cutaneous marginal zone lymphoma [1,2]. Primary cutaneous marginal zone lymphoma manifests as erythematous to violaceous nodules or plaques which may coalesce to configure papules. Frequently, lesions may be localized [1,2]. Upon microscopy, cutaneous marginal zone lymphoma (CMZL) exhibits a nodular or diffuse pattern of tumour configuration. Tumefaction manifests a ‘grenz zone’ with significant basal infiltration of neoplastic lymphocytes and cellular descent into deep seated dermis or superficial subcutaneous region, devoid of tapering. Colonization of germinal centre follicles by neoplastic lymphoma cells is enunciated although enlarged cells with significant mitotic activity are absent. However, lymphoma lacks a germinal centre immuno-phenotype [1,2]
Neoplastic monoclonal B cells demonstrate distinctive configurations as • monocytoid appearance delineating cleaved nuclei, akin to centrocytes • miniature round cells simulating chronic lymphocytic leukaemia/small lymphocytic lymphoma [1,2].
Neoplastic B cells configure dense tumour nodules, perivascular cellular infiltrates or may appear as sparse, scattered cells [1,2]. Significant quantities of plasma cells and plasmacytoid cells may be disseminated upon periphery of neoplastic nodules confined to dermis. Non-class, switched cutaneous marginal zone lymphoma frequently demonstrates tumour nodules and dense sheets of neoplastic cells pervading the dermis [1,2]. Class-switched marginal zone lymphoma preponderantly enunciates T cells along with reactive germinal B cell follicles admixed with neoplastic monoclonal B cells [1,2] Class-switched neoplasms enunciate variable plasmacytic differentiation. Previously scripted as immunocytoma, neoplasm is composed of dense aggregates of plasma cells along with bi-nucleated configurations, nuclear pleomorphism, and Dutcher bodies. Plasma cell aggregates may delineate a perivascular distribution [1,2].
Figure 1: Cutaneous marginal zone lymphoma demonstrating a nodular configuration with infiltration of post-germinal centre B lymphocytes [5].
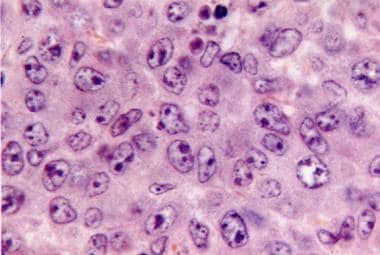
Figure 2: Cutaneous marginal zone lymphoma delineating diffuse infiltration of post germinal centre B lymphocytes with vesicular nuclei and conspicuous nucleoli [6].
International Society for Cutaneous Lymphomas (ISCL)/ European Organization of Research and Treatment of Cancer (EORTC) staging of cutaneous B cell lymphoma is designated as
Primary tumour (T)
• T1: solitary cutaneous involvement
• T1a: solitary lesion 5 centimetre diameter
• T2: regional cutaneous involvement
• T2a: all disease encompassed within 15 centimetre and 30 centimetre circular zone
• T3: generalized cutaneous involvement
• T3a: multiple lesions involving 2 non contiguous body regions
• T3b: multiple lesions involving ≥3 non contiguous body regions
Regional lymph nodes (N)
• N0: No clinical or pathological lymph node involvement
• N1: Involvement of single peripheral lymph node region which drains an area of current or preceding cutaneous tumour site
• N2: Involvement of ≥2 peripheral lymph node regions or any lymph node region which does not drain an area of current or preceding cutaneous tumour site
• N3: involvement of central lymph nodes.
Distant Metastasis
• M0: No evidence of extra-cutaneous non lymph node disease
• M1: Extra-cutaneous non lymph node disease present [2,3].
Primary cutaneous marginal zone lymphoma demonstrates kappa or lambda light chain restriction within plasma cells or plasmacytoid configurations along with detectable clonal immunoglobulin heavy chain (IGH) rearrangement obtained by polymerase chain reaction (PCR). Neoplastic monoclonal B cells exhibit a mature, post-germinal centre immuno-phenotype. Neoplastic lymphocytes are immune reactive to CD20, CD79a, BCL2, or CD43 [3,4]. Neoplastic B cells colonize germinal centre follicles and are represented within preceding germinal centre follicles, as delineated with follicular dendritic cell networks immune reactive to CD21. Neoplastic lymphocytes may circumscribe diminished germinal centres which appear immune reactive to BCL6 and CD10 [3,4]. Neoplastic Th1 dominant monoclonal B cells configuring non class switched cutaneous marginal zone lymphoma are immune reactive to CXCR3 or IgM. Infrequently, Th2 dominant, class-switched cutaneous marginal zone lymphoma appears immune reactive to CXCR3 and may demonstrate IgG or associated isotypes [3,4]. T cells may exceed quantifiable B cells and appear immune reactive to CD4, PD-1, or BCL6. Primary cutaneous marginal zone lymphoma is immune non reactive to BCL6, CD10, cyclinD1, or CD5. Immune-reactive CD5 cells are infrequently discerned [3,4]. Primary cutaneous marginal zone lymphoma requires segregation from neoplasms such as chronic lymphocytic leukemia/small lymphocytic lymphoma, cutaneous lymphoid hyperplasia, diffuse large B cell lymphoma, follicular lymphoma, lymphoplasmacytic lymphoma, mantle cell lymphoma, myeloma(plasmacytoma), primary cutaneous CD4+ small/ medium sized pleomorphic T cell lymphoproliferative disorder, primary cutaneous follicle centre cell lymphoma, pseudolymphomatous folliculitis, splenic lymphoma or timid lupus erythematosus [3,4]. Besides, primary cutaneous marginal zone lymphoma necessitates demarcation from cutaneous component of lymphoma of extra-cutaneous origin which can be achieved with clinical concurrence as a comprehensive history, physical examination, complete blood count, and precise evaluation with imaging techniques [3,4]. Serological assay for infection with Borrelia burgdorferi may be beneficially adopted for differentiating reactive lymphoid hyperplasia from cutaneous marginal zone lymphoma within pertinent geographic regions such as Europe [3,4]. Light chain restriction may be observed with in situ hybridization (ISH) or pertinent immunohistochemistry. Polymerase chain reaction (PCR) exhibits clonally rearranged immunoglobulin heavy chain gene, in contrast to reactive cutaneous lymphoid hyperplasia [3,4]. Localized instances of primary cutaneous B cell lymphoma confined to various cutaneous surfaces may be appropriately treated with surgical excision, radiotherapy, or intralesional steroid administration [3,4]. Additionally, withdrawal of offending agent inducing cutaneous marginal zone lymphoma as an antihistamine or antidepressant may be advantageous [3,4]. Primary cutaneous marginal zone lymphoma demonstrates superior 5-year overall survival of > 95%. A subset (4%) of neoplasms are associated with extra-cutaneous dissemination [3,4]. Tumour relapse occurs in <50% of neoplasms and is commonly encountered in neoplasms with multifocal cutaneous involvement. Exceptionally, metamorphosis into an aggressive, blastic, or large cell lymphoma, akin to splenic B cell lymphoma may ensue [3,4].
REFERENCES
1. Ronchi A, Sica A, Vitiello P, Franco R. (2021). Dermatological Considerations in the Diagnosis and Treatment of Marginal Zone Lymphomas. Clin Cosmet Investig Dermatol. 14:231- 239.
2. Fava P, Roccuzzo G, Alberti-Violetti S, Grandi V, Pileri A, Pimpinelli N, et al. (2022). Cutaneous B-cell lymphomas: Update on diagnosis, risk-stratification, and management. Presse Med. 51(1):104109.
3. Vitiello P, Sica A, Ronchi A, Caccavale S, Franco R, Argenziano G. (2020). Primary Cutaneous B-Cell Lymphomas: An Update. Front Oncol. 10:651.
4. Watts-Santos A, Cuellar-Barboza A, Jair Ramos-Cavazos C, Villarreal-Martinez A, Ocampo-Candiani J, Herz-Ruelas ME. (2021). Indurated Plaques on the Legs: Think Lymphoma. Acta Dermatovenerol Croat. 29(2):114-115.
 Abstract
Abstract  PDF
PDF