Past Issues
The Effect of Treprostinil on Metabolic Profile in a Patient with Secondary Pulmonary Hypertension from Chronic Lung Disease
Thyyar M Ravindranath1,*, Caylin Hughes2
1Division of Pediatric Intensive Care Unit, Department of Pediatrics, Morgan Stanley Children’s Hospital of New York-Presbyterian, Columbia University Medical Center, New York, New York, USA
2Pediatric Nutrition Support Specialist, New York-Presbyterian/Morgan Stanley Children's Hospital, New York, New York, USA
Received Date: May 16, 2023
Publication Date: June 12, 2023
Citation: Ravindranath Thyyar M, et al. (2023). The Effect of Treprostinil on Metabolic Profile in a Patient with Secondary Pulmonary Hypertension from Chronic Lung Disease. Cases. 2(2):12.
Copyright: Ravindranath TM, et al. © (2023)
*Corresponding Author: Thyyar M. Ravindranath, MD, Division of Pediatric Intensive Care Unit, Department of Pediatrics, Morgan Stanley Children’s Hospital of New York-Presbyterian, Columbia University Medical Center, New York, New York, USA; E-mail: [email protected]
ABSTRACT
The effects of medications used in the Pediatric Intensive Care Unit (PICU) on resting energy expenditure (REE) are not well known. Studying the effects of medication on REE would help to tailor energy needs of critically ill children more precisely. The use of Indirect Calorimeter (IC) has helped to measure REE accurately in critically ill patients. We describe the effect of continuous infusion of prostaglandin, treprostinil on REE and other parameters measured by IC in a child with pulmonary hypertension secondary to chronic lung disease and giant omphalocele. It appears that the effects of treprostinil on parameters measured by IC were dose dependent. This case report illustrates the importance of accurately measuring REE to modify nutritional therapy in critically ill children in PICU.
Keywords: Pediatric Intensive Care Unit, Resting Energy Expenditure, Indirect Calorimeter, Pulmonary Hypertension, Treprostinil, Chronic Lung Disease
INTRODUCTION
It is important to understand the effects of prescribed drugs on metabolism in order to tailor the nutritional prescription for critically ill children in the PICU. We describe a case of an infant with chronic lung disease and pulmonary hypertension secondary to omphalocele. This report narrates the effects of treprostinil [1,2], a pulmonary vasodilator prostaglandin, during a continuous subcutaneous infusion used to control pulmonary hypertension with a metabolic profile obtained via an indirect calorimeter (IC) (CCM Express, MC Diagnostics, St. Paul, MN). Treprostinil is a prostanoid that targets the prostacyclin pathway resulting in vasodilation, inhibition of smooth muscle proliferation and platelet aggregation [3]. The systemic effect of treprostinil may modify energy metabolism in a dynamic manner providing a challenge for caretakers to provide appropriate nutritional support.
MATERIALS AND METHODS
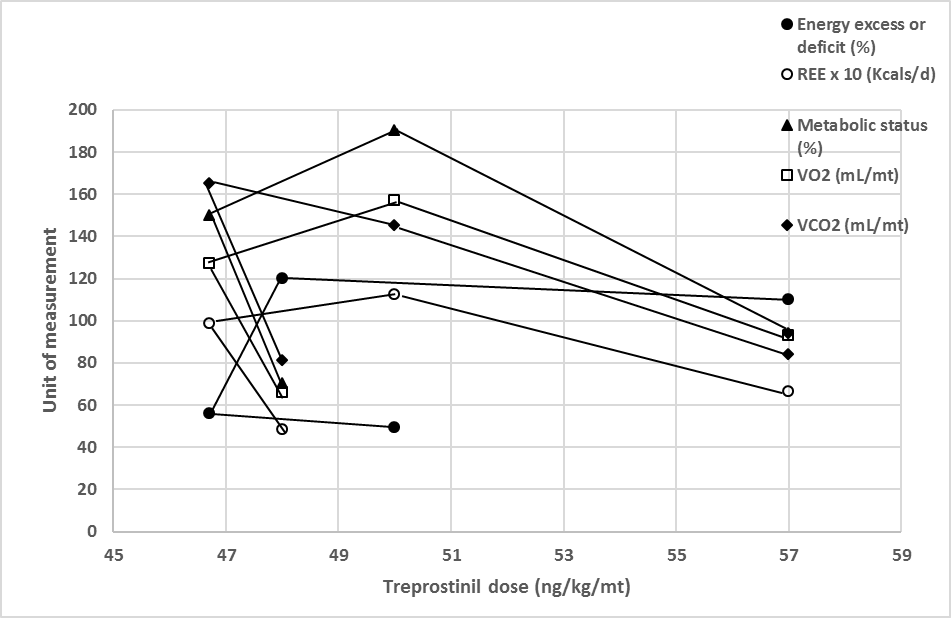
Consent was obtained from one of the legal guardians before performing IC. A 19-month-old full term male was born with a giant omphalocele (unrepaired in silo) that contained spleen, liver and stomach. He also had left renal agenesis, vertebral anomalies. He underwent tracheostomy at 2 months of age and subsequently was placed on V-V ECMO for severe respiratory failure at 13 months of age with concurrent direct implantation of scimitar vein into the left atrium, repair of left upper pulmonary vein stenosis and fenestrated patch closure of the atrial septal defect (ASD). He stayed in the PICU for more than a year and during his stay, underwent IC at 6, 8, 10 and 12 months while in the PICU to assess his calorie needs. A Steady state was attained for at least 5 minutes. The figure shows various metabolic parameters that included resting energy expenditure (REE), metabolic status, oxygen consumption (VO2), carbon dioxide production (VCO2) as well as energy excess. Dividing measured REE with calculated or predicted energy expenditure obtained from Schofield equation for age provided his metabolic status [4]. Metabolic status was classified as normo (90-110%), hypo (<90%) and hypermetabolic (>110%) states. Dividing provided REE with measured REE (5) resulted in energy excess. Measurement of IC parameters took place at different doses of continuous treprostinil infusion given by subcutaneous route at 6, 8, 10 and 12-month time points. Both VCO2 and VO2 were elevated above normal. His pro-BNP level was elevated to 3227 at the 6-month time point. Mild elevation in TSH (6-month time point), CRP (6 & 8-month time points) were noted, core body temperature (at all-time points) and lactate level (at 6, 8, 10-month time points) were normal (Table). ECHO at 17 months of age showed a small secundum ASD, moderate to severe right ventricular hypertrophy, mild to moderate dilated right ventricle, and severe right ventricular hypertension. Two left pulmonary veins were identified, and the left upper pulmonary vein had flow acceleration with a mean gradient of 4 mmHg and the right veins connection had mild flow acceleration with a mean gradient of 6 mmHg (normal gradient of 0).
DISCUSSION
Nutrition support of critically ill pediatric patients is complicated. The energy requirement changes from time to time depending on the state of critical illness [6]. Predictive equations used for critically ill PICU patients can under or overestimate energy needs necessitating the use of IC to measure energy requirement accurately [7]. Even a single intermittent measurement may not adequately assess energy needs, requiring multiple measurements during a patient’s PICU stay. Many factors influence IC measurements including administered medications in PICU [8]. In this case report, we documented the effect of the pulmonary vasodilator treprostinil on various parameters measured by IC. There appeared to be a dose response effect of treprostinil on REE, metabolic status, VO2, VCO2 as shown in the figure at 6, 8, 10 and 12 months of stay in PICU. An increase in REE in a hypometabolic state indicated that the change in REE was unrelated to his metabolic status. Although initially there was a decrease in REE at a higher treprostinil dose, which was followed by an increase in REE at a lower dose, with a subsequent increase in REE at a higher dose with decreased REE on further increases in treprostinil dose. Our patient’s metabolic status changed from normometabolic to hypometabolic at the first two time points followed by a hypermetabolic state on the last two time points, highlighting the dynamic nature of energy metabolism. Despite adjusting the nutrition prescription for the measured REE, there was an energy deficit initially followed by excess, which did not seem to influence other measured parameters. The change in energy measurement may be due to the dynamic nature of energy requirement in our patient, suggesting the need for more frequent measurement of REE by IC. Our patient had no fever, thyroid dysfunction, significant elevation in white blood cell count within 48 hours or days of IC evaluation (table), suggesting the absence of infection or thyroid dysfunction as responsible factors for the change in metabolic status. However, there was a mild elevation in CRP at the first two time points, suggesting an inflammatory response likely related to right heart failure as suggested by an elevated pro-BNP as well as troponin leak noted prior to first IC measurement (table). We did not encounter other factors that promote an elevated VO2 such as surgery, sepsis, shivering, pain, agitation, and physical therapy prior to or at the time of IC measurement in our patient [9]. Etiologies for increased VCO2 include hypermetabolism, overfeeding with excessive carbohydrate and acidosis [10]. The figure shows that there was no relationship between metabolic status and VCO2. The VCO2 was above normal at all-time points irrespective of the metabolic status. Overfeeding with excessive carbohydrate can induce elevation in VCO2, however our patient’s calorie intake was 49% and 56% of the measured caloric intake at the first 2 time points with carbohydrate constituting 53% and 48% of total calories, respectively. This coincided with increasing VCO2 at the 1st time and 2nd time point which appeared to be unrelated to the carbohydrate load. However, VCO2 decreased at 3rd and 4th time points despite increased provision of energy at 120% and 110% above his measured energy requirement with carbohydrate constituting 42% and 40% of total calories provided, respectively. This suggests that overfeeding with excessive carbohydrate provision was not the reason for elevated VCO2. No evidence of organic acidosis existed in our infant excluding other known reasons for elevated VCO2. Our patient was also on sildenafil as well as bosentan by nasogastric tube, but not nitric oxide for moderate to severe pulmonary hypertension. Although our patient had an initial elevated pro-BNP and normal lactate levels at the first three of the four time points, suggested sufficient tissue perfusion with an adequate cardiac output.
Table: Shows measured laboratory parameters.
|
Laboratory Parameter on or within 48 hours of IC measurement (normal value) |
IC measurement following admission (months) |
|||
|
6 |
8 |
10 |
12 |
|
|
Temperature (?C) |
37.4 |
37.8 |
37.5 |
37.4 |
|
WBC (5.98-13.5 x 10 (3)/uL) |
12.6 |
10.6 |
15.1* |
13.5** |
|
Lactate (0.5-1.6 mmol/L) |
1.1 |
1.5 |
1.5 |
1.5*** |
|
Albumin (3.9-5.2 g/dL) |
4.7 |
3.6 |
4.2 |
5.1 |
|
CRP (0-10 mg/L) |
12.9 |
35.5 |
11.4# |
11.4## |
|
Carbohydrate (% of total Kcal) |
53 |
48 |
42 |
40 |
|
Pro-BNP (32-675 (pg/mL) |
1970 |
1460 |
602### |
3277 |
|
Other laboratory parameters |
||||
|
Ferritin (30-400 ng/mL) |
78.6 |
|||
|
TSH (0.4-4.8 mU/L) |
6.75 |
|||
|
Troponin (<0.01 ng/mL) |
1.3† |
|||
Table shows measured laboratory parameters.
*Refers to hours prior to IC measurement: *72, **25, ***10
# Days prior to IC measurement: #7, ##24, ###3
†Days prior to IC measurement: 48
Figure: Shows parameters measured using indirect calorimeter. REE: Resting Energy Expenditure.
REE 10 x indicates value on Y-axis multiplied by 10 gives total REE. VO2: Oxygen consumption, VCO2: CO2 production.
Several limitations exists in our case presentation. It was a single case description, and we were unable to perform IC during earlier time points. Evidence of right heart failure was noted in our case which may have contributed to the inflammation as evidenced by elevation in CRP and may not be related to treprostinil. It was also difficult to exclude the effects of other drugs including sildenafil and bosentan on measured metabolic profile.
Conclusion: Despite certain limitations in our case report, it appears that medications such as tresprostinil used to treat complex medical conditions, may have profound effects on energy expenditure requiring constant attention and multiple IC measurements to tailor nutritional support for these children. This case illustrates the need for a larger study to document the effect of continuous infusion of a pulmonary vasodilator on energy needs of children with pulmonary hypertension.
CONFLICTING INTEREST DECLARATION
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or Publication of this article.
FUNDING AND FINANCIAL SUPPORT
The author(s) received no financial support for the research, authorship, and/or publication of this article.
CONTRIBUTORS
Thyyar M Ravindranath contributed to the conception and design of the research; Caylin Hughes contributed to the acquisition and Thyyar M Ravindranath and Caylin Hughes contributed to the analysis of the data; Thyyar M Ravindranath and Caylin Hughes contributed to the interpretation of the data; and Thyyar M Ravindranath drafted the manuscript.
ETHICAL CLEARANCE
Clinicians at our institution are not required to obtain IRB approval for case reports of a single patient.
REFERENCES
- Ablonczy L, Tordas D, Kis E, Szatmari A. (2018). Use of subcutaneous treprostinil in pediatric pulmonary arterial hypertension-Bridge-to-transplant or long-term treatment? Pediatr Transplant. 22(2):1-5.
- Levy M, Del Cerro MJ, Nadaud S, Vadlamudi K, Colgazier E, Fineman J et al. (2018). Safety, efficacy and management of subcutaneous treprostinil infusions in the treatment of severe pediatric pulmonary hypertension. Int J of Cardiol. 264(1):153-157.
- Del Pozo R, Hernandez Gonzalez I, Escribano-Subias P. (2017). The prostacyclin pathway in pulmonary arterial hypertension: a clinical review. Expert Rev of Respir Med. 11(6):491–503.
- Smallwood CD, Mehta NM. (2012). Accuracy of Abbreviated Indirect Calorimetry Protocols for Energy Expenditure Measurement in Critically Ill Children. J Parenter Enteral Nutr. 36(6):693-699.
- Kerklaan D, Hulst JM, Verhoeven JJ, Verbruggen SC, Joosten KF. (2016). Use of Indirect Calorimetry to Detect Overfeeding in Critically Ill Children: Finding the Appropriate Definition. J Pediatr Gasteroenterol Nutr. 63(4):445-450.
- Mehta NM, Bechard LA, Leavitt K, Duggan C. (2009). Cumulative Energy Imbalance in the Pediatric Intensive Care Unit: Role of Targeted Indirect Calorimetry. J Parenter Enteral Nutr. 33(3):336-344.
- Coss-Bu JA, Jefferson LS, Walding D, David Y, Smith EO, Klish WJ. (1998). Resting energy expenditure in children in a pediatric intensive care unit: comparison of Harris-Benedict and Talbot predictions with indirect calorimetry values. Am J Clin Nutr. 67(1):74-80.
- Mehta NM, Smallwood CD, Graham RJ. (2014). Current Applications of Metabolic Monitoring in the Pediatric Intensive Care Unit. Nut Clin Pract. 29(3):338-347.
- McLellan SA, McClelland DBL, Walsh TS. (2003). Anemia and red blood cell transfusion in the critically ill patient. Blood Rev. 17(4):195-208.
- Weinberger SE, Schwartzstein RM, Weiss JW. (1989). Hypercapnea. N Engl J Med. 321:1223-1231.
 Abstract
Abstract  PDF
PDF